胡務亮 Wuh-Liang Hwu

國立臺灣大學 醫學院小兒科教授
學歷
- 國立臺灣大學醫學院分子醫學研究所博士(1997)
- 國立臺灣大學醫學院醫學士(1984)
經歷
- 國立臺灣大學醫學院小兒科教授(2010/7 ~ 迄今)
- 臺大醫院小兒部副主任(2018/8 ~ 2021/7)
- 臺大醫院基因醫學部主任(2005/8 ~ 2013/7)
個人勵志銘
Do something worthwhile and meaningful.
成功研發AADC 缺陷基因治療 從臨床試驗至新藥核准
I developed new diagnoses and treatments for two rare diseases – Pompe disease and aromatic L-amino acid decarboxylase (AADC) deficiency. I am the first to introduce secondgeneration newborn screening, employing tandem mass spectrometry, in Taiwan, and this technology significantly broadens the scope of screening for newborn babies. In 2005, I launched the first-in-the-world newborn screening for Pompe disease in Taiwan. This project demonstrated the feasibility of measuring acid α-glucosidase, a lysosomal enzyme, activity from dried blood spot. After that, we can diagnose Pompe disease in newborn infants. The success of this program changes the global standards of diagnosis and treatment for Pompe disease. A few years later, Pompe disease was included in the Recommended Uniform Newborn Screening Panel in USA. Currently, more lysosomal storage diseases are included in newborn screening, and certainly my group is still leading in this field, including early-treatment clinical trials for lysosomal storage diseases.
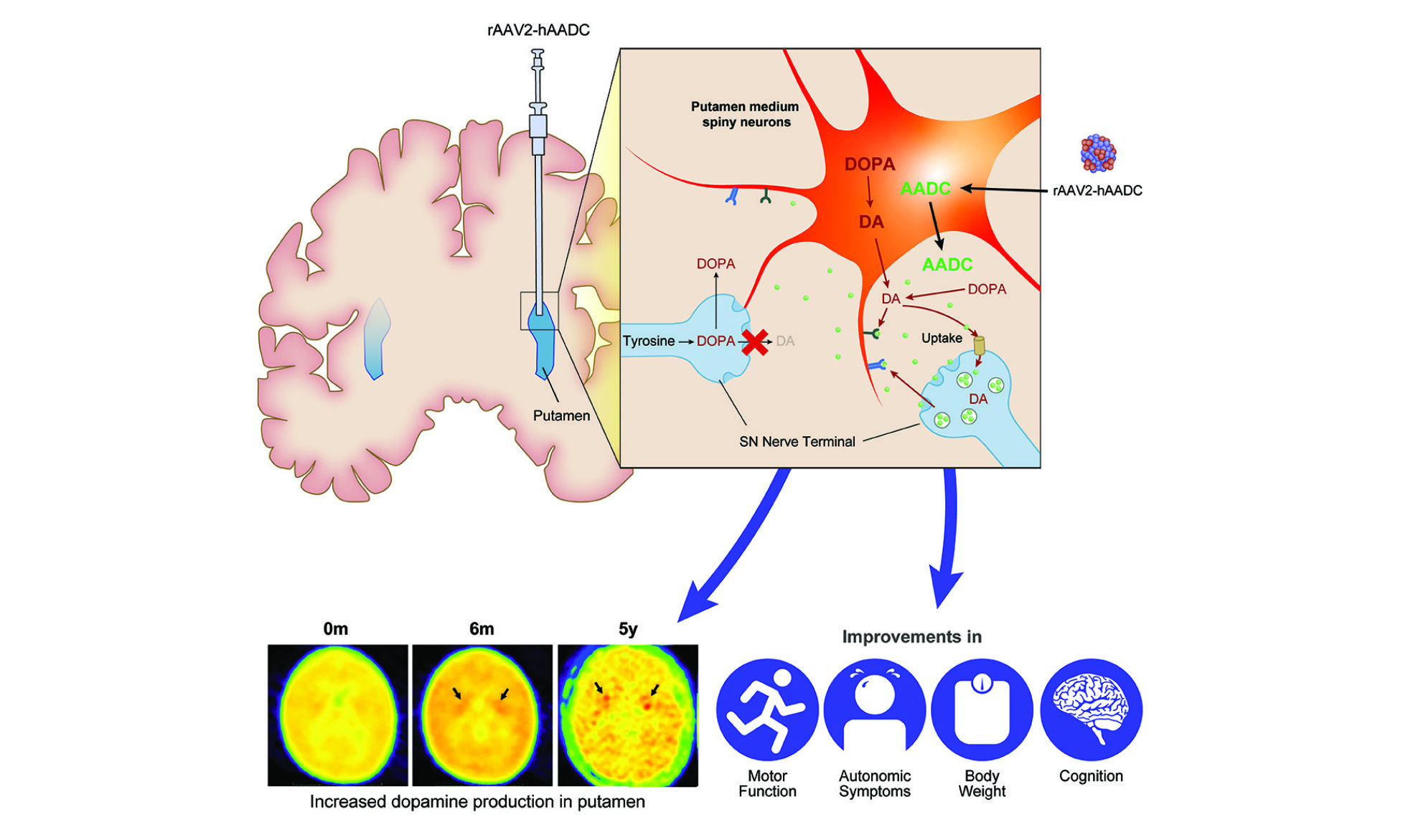
I started to compose a gene therapy for an ultrarare disease –AADC deficiency since 2007. This gene therapy involves bilateral intraputaminal injection of adeno-associated viral vector type 2 (AAV2) that expresses human AADC cDNA, through stereotactic brain surgery. The first dosing was performed in 2010. We performed several clinical trials, including an initial compassionate use study, a phase I/II trial, and a phase IIb trial. We published a series of papers in Science, Lancet, and Molecular Therapy. This 2technology was licensed to Agilis Biotherapeutics in 2015, and Agilis was acquired by PTC Therapeutics in 2018. This gene therapy was approved by European Medicines Agency (EMA) as a new drug on Jul, 18, 2022. This is also the first successful central nervous system (CNS) gene therapy for single gene diseases. In addition, I have created a AADC deficiency mouse model for more translational studies.
After several years of hard work, I finally developed a new drug for a rare neurological disease that has no treatment in the past. This is certainly a big step for biomedical industry in Taiwan.
得獎感言
This award is a great encouragement to me and to my group. Thanks for my colleagues and team members, for those who have helped and supported us, and more deeply, for the contribution of patients and their families. We will certainly keep on finding new treatments for patients with rare diseases.

