鄒 倫 Lun Kelvin Tsou

財團法人國家衛生研究院 生技與藥物研究所副研究員
學歷
- 美國北卡羅來納大學教堂山分校學士(2002)
- 美國耶魯大學博士(2007)
- 美國洛克菲勒大學/ 博士後研究員(2012)
經歷
- 國家衛生研究院生技與藥物研究所研究員(2022/10 ~ 迄今)
- 國家衛生研究院生技與藥物研究所副研究員(2017/10 ~ 2022/9)
- 國家衛生研究院生技與藥物研究所助研究員(2012/9 ~ 2017/9)
個人勵志銘
"Try not to become a man of success. Rather become a man of value." -Albert Einstein
研發小分子抗癌藥物複合體 展開人體一期臨床試驗
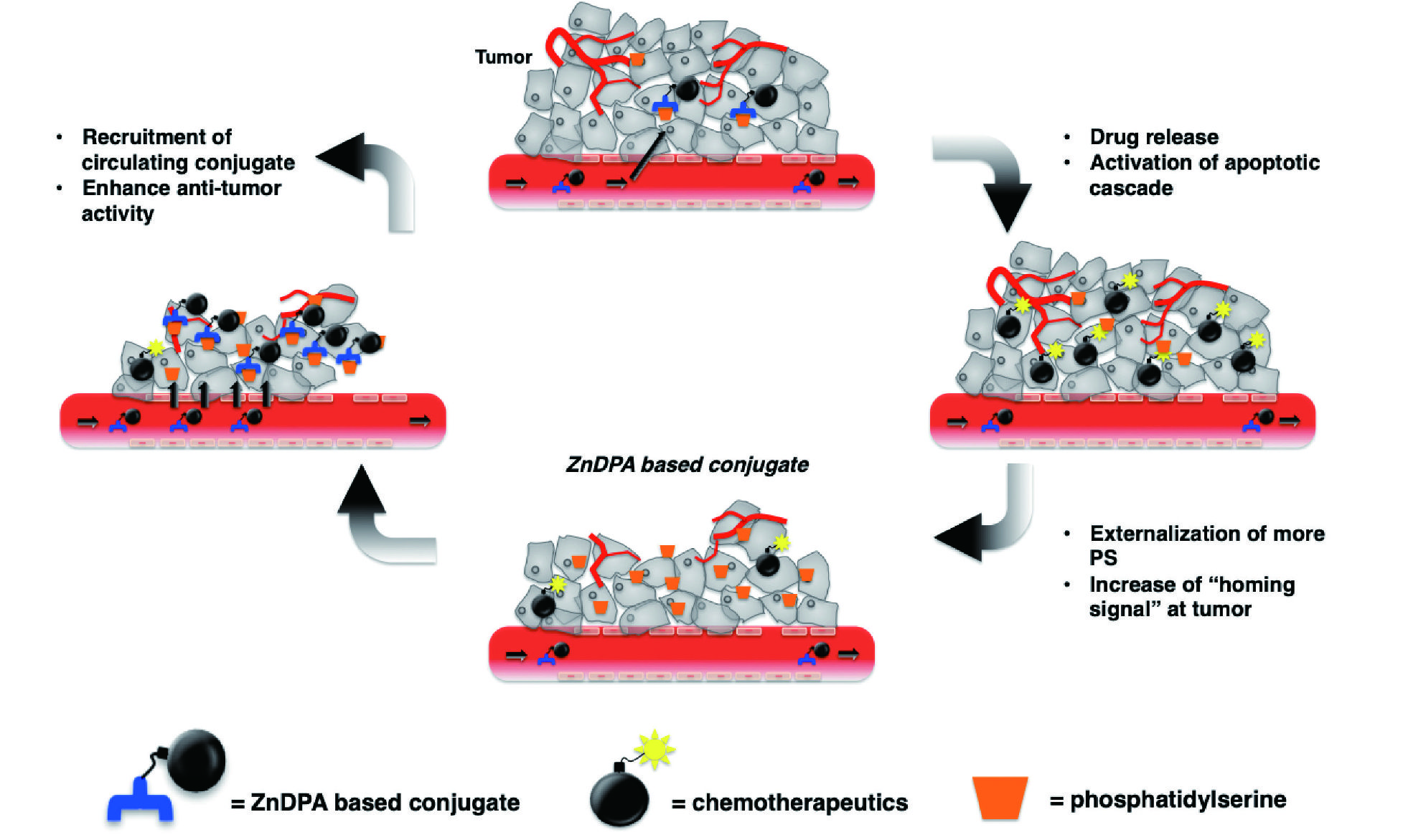
My scientific endeavor started with undergraduate research in professor Marcey Waters’ lab at UNC-Chapel Hill to investigate the magnitude of noncovalent cation - π interactions in stabilizing the folding of an α-helix. As a graduate student, I carried out the design and synthesis of several protein-protein interaction disruptors in professor Andrew D. Hamilton’s lab at Yale University. Through collaboration with Dr. Yung-Chi Cheng, I also identified novel macrocyclics with potent dual anti-HIV and anti-HCV activities. As a postdoctoral fellow with professor Howard C. Hang at Rockefeller University, I developed chemical tools targeting type III secretion systems in bacterial pathogenesis. At NHRI, my lab has designed and synthesized a series of small molecule drug conjugates (SMDCs) for the treatment of cancer. Through a facile and cost-effective synthesis, we obtained potent Zinc (II) Dipicolylamine (ZnDPA)-based small molecule drug conjugate for the treatment of phosphatidylserine-expressing solid tumors. In collaboration with the labs of Dr. Tsu-An Hsu, Dr. Teng-Kuang Yeh, and Dr. Chiung-Tong Chen, our work culminated several linkers with good in vivo plasma stability that harnessed an enzymatically cleavable site to allow the release of the payload in the tumor microenvironment. Moreover, my lab has developed several synthetic strategies to increase the diversity and solubility of these linkers , and the incorporation of different cytotoxic payloads in these conjugates. The designed integration of appropriate targeting moiety with the drug’s pharmacological response is a promising strateg y for de veloping next-generation cancer therapeutics. In particular, DBPR115 was selected as the development candidate, and technology transferred to a Taiwanbased biotech company in 2016. In 2021, with the completion of the preclinical studies, DBPR115 was successfully granted IND status by US-FDA and Taiwan FDA and has entered Phase I clinical trial in Taiwan.
得獎感言
It’s an honor to receive this award and recognize by the respective NSTC scientific committee. I want to thank former director Dr. Yu-Sheng Chao for taking a chance on the new kid and supporting me with a highly motivated cast to work on challenging projects. With eminent help from many colleagues, Dr. Kak-Shan Shia, Dr. Hsing-Pang Hsieh, Dr. Chiung-Tong Chen, and Dr. Jang-Yang Chang, several projects have taken off with promising results under steady financial support. I also want to thank former director Dr. Chuan Shih for challenging my team to generate a competitive drug candidate and providing great guidance throughout the technology transfer process. I hope this award can render a great testament to integrative teamwork between different scientific disciplines and a humble reminder of the great teamwork/project management environment that I have been immersed in since my arrival at IBPR.

